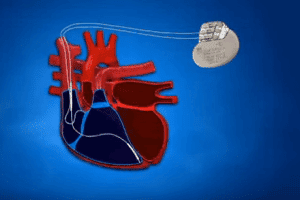
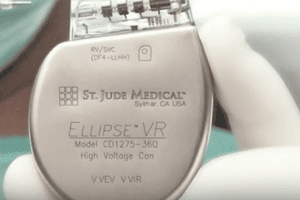
Hacking Risk Sparks 465,000 St. Jude Pacemakers Recall
Patients vulnerable to a software glitch The U.S. Food and Drug Administration (FDA) is warning that over 465,000 patients who had St. Jude pacemakers implanted may be vulnerable to a [...]