
Parker Waichman LLP is Reviewing Cases of Risperdal Gynecomastia and Offers You a Free Case Review Parker Waichman LLP learned of a recent court ruling which may pave the way for additional claims for punitive damages against Risperdal (risperidone) manufacturers Janssen Pharmaceuticals and Johnson & Johnson. The ruling handed down February 8, 2018, in the […]
Parker Waichman LLP learned of a recent court ruling which may pave the way for additional claims for punitive damages against Risperdal (risperidone) manufacturers Janssen Pharmaceuticals and Johnson & Johnson. The ruling handed down February 8, 2018, in the Superior Court of Pennsylvania permits nearly 6,400 plaintiffs to pursue punitive damages against Risperdal’s manufacturers after a lower court ruled that the plaintiffs not be allowed to receive punitive damages in this situation.
The Pennsylvania Superior Court’s decision could force Janssen and Johnson & Johnson to alter their strategy in defending Risperdal cases and settle, rather than force them to trial. However, the history of these cases might suggest otherwise. Janssen has dug in hard and taken a position that they are not responsible. Part of that fight includes Janssen taking the stance that New Jersey law should apply in Pennsylvania. A favorable ruling on that front would have benefitted Janssen greatly. New Jersey law would have prevented the plaintiffs from receiving punitive damages because the U.S. Food and Drug Administration (FDA) approved Risperdal. However, the Superior Court stated that Wisconsin law applied. Wisconsin law does not preclude a plaintiff from recovering punitive damages from a drug maker who obtained FDA approval for its drug.
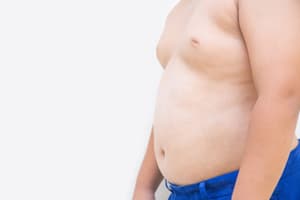
Janssen and Johnson & Johnson may be liable for failing to warn about significant, life-altering side effects, like pre-pubescent males developing gynecomastia, which lasted into adulthood. The mental anguish for these young men who now must face the sometimes cruel and awkward high school and college years having to explain why they have female breast tissue is often excruciating. The sad part is that many treating physicians would have prescribed a different medication for their patients if the doctors who prescribed the medication themselves knew about the possible side effect that gynecomastia could develop. Unfortunately, Janssen and Johnson & Johnson failed to disclose the relevant testing information to physicians, who could have appropriately advised their patients in turn. Instead, many young men are scarred for life.
If you or a loved one took Risperdal and suffered significant side effects like developing gynecomastia, you might be eligible to bring a lawsuit for damages, including punitive damages, for legal claims against Janssen and Johnson & Johnson. The Risperdal Gynecomastia lawsuit lawyers with Parker Waichman LLP want to help you recover financial compensation for the medical bills, future medical costs, as well as the physical and psychological pain and suffering you or your loved one endured at the hands of Janssen and Johnson & Johnson.
Parker Waichman LLP is a national law firm who has developed a reputation for excellence as plaintiffs’ counsel who battles monolithic drug companies like Janssen and Johnson & Johnson and recovers substantial damages for their clients. Parker Waichman LLP’s long history of providing high-quality legal services to their clients has led to recovering over $2 billion in damages. Their track record of success says it all. Parker Waichman LLP is the drug defect law firm you can trust to put you in the best legal position to obtain a substantial settlement or damage award after trial.
The FDA must approve medications for specific uses before the drug maker can market its product in the U.S. The FDA permitted Janssen to sell Risperdal to adults and children in varying doses for Schizophrenia, Bipolar disorder, and Autism Spectrum Disorder. Once the drug entered the market, other so-called “Off-label” uses have become prevalent. Off-label uses for Risperdal include treatment and maintenance of adult and childhood:
Risperdal treats some of these psychological diseases more efficiently than others. However, Risperdal is a drug frequently prescribed by psychiatrists and treating physicians based on the combined label and off-label uses. The numerous uses make Risperdal a versatile drug. As a result, a large number of people in the population encounter Risperdal on a daily basis. The sheer volume of people who come in contact with Risperdal on a daily basis should be a concern from Janssen and Johnson & Johnson.

Gynecomastia is a side effect of taking Risperdal which is the subject of thousands of civil complaints pending against Janssen in the Court of Common Pleas in Pennsylvania. Over 6,000 cases are now pending in Pennsylvania. One case, in particular, blazed a trail to giving plaintiffs an opportunity to pursue punitive damages.
In that case, a young man who was on the Autism Spectrum experienced a sharp pain in the left nipple. The young man’s mother took him to his primary care physician who, in turn, was able to diagnose the boy with gynecomastia. The next step was a referral to a plastic surgeon. The boy endured a double mastectomy. He recovered from the surgery well but still had permanent scars left behind that at times, caused him a great deal of pain. To make matters worse, the young man was picked on by other kids in school because of the breast growth.
After the trial, a jury awarded the plaintiff $500,000.00 in compensatory damages. The judge did not allow the case to go to the jury on the issue of punitive damages. At trial, the plaintiffs proved that the Risperdal raised prolactin levels in the body and that Janssen and Johnson & Johnson were aware of those risks. The plaintiffs showed that the defendant knew that elevated prolactin levels could cause gynecomastia. Despite that knowledge, the defendants never put that warning on the label until the latter part of 2006, even after using the drug for adolescent and child use for at least four years.
Janssen held such detailed knowledge about the connection between Risperdal and gynecomastia that they could pinpoint when the deformity might begin to take shape. Studies found that as many as 12.5% of all young man taking Risperdal entered the initial stages of female breast growth after taking the drug for anywhere between 8 to 12 weeks. Notwithstanding, Janssen changed statistical protocols and later stated that they did not have any statistically significant data to demonstrate that Risperdal consumption would lead to gynecomastia. Consequently, Janssen failed to report the statistics to the FDA and did not publish the stats after the FDA approved to prescribe Risperdal to children with autism.
Janssen did produce warning labels that indicated the increased chance of a teenage boy getting gynecomastia. The Superior Court of Pennsylvania found that there was compelling evidence to suggest that Janssen significantly understated the significance of the findings possessed by Janssen.
The plaintiff’s treating physician was a compelling witness. The doctor testified that if he were aware that Risperdal had a higher risk of leading to gynecomastia, then he would have prescribed another drug and not Risperdal. That testimony was significant to the plaintiff. Under the “learned intermediary” doctrine, the law allows the plaintiff to rely on an expert’s opinion, such as a medical doctor, to help prove that the drug manufacturer failed to warn.
Many law firms claim that they have the edge when it comes to having superior knowledge or the key to success when bringing a claim against drug manufacturers. Parker Waichman LLP’s drug defect lawyers have proven time and time again in court and through settlements that they have what it takes to make a difference for you. Call Parker Waichman LLP’s drug defect lawyers today at 1-800-YOURLAWYER (1-800-968-7529) or use our contact form to speak with us about your Risperdal legal claim.


