
Fosamax Femur Fracture Lawsuits. A woman who claims to have suffered a femur fracture as a result of taking the bone loss drug Fosamax is the latest to file a lawsuit against the makers of the drug. The national law firm of Parker Waichman LLP, which represents other victims of Fosamax injuries across the […]
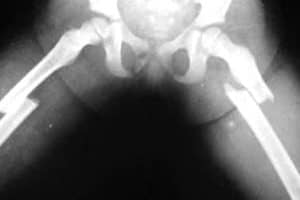
Fosamax Femur Fracture Lawsuits. A woman who claims to have suffered a femur fracture as a result of taking the bone loss drug Fosamax is the latest to file a lawsuit against the makers of the drug.
The national law firm of Parker Waichman LLP, which represents other victims of Fosamax injuries across the country, has filed the lawsuit in Superior Court of New Jersey, Atlantic County Law Division against Merck & Co., as well as other unnamed possible manufacturers of the drug.
The woman represented in the lawsuit states she began taking Fosamax, one of the most popularly prescribed bisphosphonate drugs available in the U.S., in December 2004 to prevent bone loss caused by osteoporosis. She suffered an atypical femur fracture in September 2009, less than five years after starting the drug treatment.
This side effect is the basis for numerous other lawsuits across the country and like those others, the Plaintiff claims she was never warned of the dangers of this rare and painful bone fracture risk. The femur is considered one of the most difficult bones in the body to break and others taking Fosamax have also reported suffering this side effect while taking the drug.
Although Fosamax and other bisphosphonate drugs are designed to increase bone density, this and similar lawsuits claim this doesn’t necessarily reduce the risk of fractures caused by the drug. In fact, they allege that bisphosphonate drugs make bones “mineralized and brittle,” according to a release from Parker Waichman announcing the filing of the lawsuit on June 4 in New Jersey. It also claims Merck and other companies failed to do post-market
The femur fracture has altered the life of the Plaintiff in this case and she seeks damages for “severe mental and physical pain and suffering, permanent injuries and emotional distress, economic loss due to medical expenses and living related expenses due to a new lifestyle.”
The Food and Drug Administration only placed a warning specific to the risk of atypical femur fractures on the labels of Fosamax and other bisphosphonate drugs in 2010, long after the woman represented in this lawsuit claims to have started taking the drug and a year after suffering this serious injury.
Two recent post-market analyses of Fosamax and bisphosphonate safety indicated that these drugs serve very little clinical benefit after five years of taking them. That study was conducted by the FDA and published by the New England Journal of Medicine. In the same month, the Archives of Internal Medicine conducted a review of women who had suffered atypical femur fractures and found that 82 percent of them had taken a bisphosphonate drug like Fosamax.
woman who claims to have suffered a femur fracture as a result of taking the bone loss drug Fosamax is the latest to file a lawsuit against the makers of the drug.
The national law firm of Parker Waichman LLP, which represents other victims of Fosamax injuries across the country, has filed the lawsuit in Superior Court of New Jersey, Atlantic County Law Division against Merck & Co., as well as other unnamed possible manufacturers of the drug.
The woman represented in the lawsuit states she began taking Fosamax, one of the most popularly prescribed bisphosphonate drugs available in the U.S., in December 2004 to prevent bone loss caused by osteoporosis. She suffered an atypical femur fracture in September 2009, less than five years after starting the drug treatment.
This side effect is the basis for numerous other lawsuits across the country and like those others, the Plaintiff claims she was never warned of the dangers of this rare and painful bone fracture risk. The femur is considered one of the most difficult bones in the body to break and others taking Fosamax have also reported suffering this side effect while taking the drug.
Although Fosamax and other bisphosphonate drugs are designed to increase bone density, this and similar lawsuits claim this doesn’t necessarily reduce the risk of fractures caused by the drug. In fact, they allege that bisphosphonate drugs make bones “mineralized and brittle,” according to a release from Parker Waichman announcing the filing of the lawsuit on June 4 in New Jersey. It also claims Merck and other companies failed to do post-market
The femur fracture has altered the life of the Plaintiff in this case and she seeks damages for “severe mental and physical pain and suffering, permanent injuries and emotional distress, economic loss due to medical expenses and living related expenses due to a new lifestyle.”
The Food and Drug Administration only placed a warning specific to the risk of atypical femur fractures on the labels of Fosamax and other bisphosphonate drugs in 2010, long after the woman represented in this lawsuit claims to have started taking the drug and a year after suffering this serious injury.
Two recent post-market analyses of Fosamax and bisphosphonate safety indicated that these drugs serve very little clinical benefit after five years of taking them. That study was conducted by the FDA and published by the New England Journal of Medicine. In the same month, the Archives of Internal Medicine conducted a review of women who had suffered atypical femur fractures and found that 82 percent of them had taken a bisphosphonate drug like Fosamax.
The personal injury attorneys at Parker Waichman LLP offer free, no-obligation case evaluations. For more information, fill out our online contact form or call 1-800-YOURLAWYER (1-800-968-7529).


