
Infusion systems has now been deemed a Class I recall. Medtronic Inc.’s January communication regarding problems with its SynchroMed and IsoMed infusion systems has now been deemed a Class I recall by the Food & Drug Administration (FDA). A Class I recall indicates a situation in which there is a reasonable probability that use of the […]
Infusion systems has now been deemed a Class I recall. Medtronic Inc.’s January communication regarding problems with its SynchroMed and IsoMed infusion systems has now been deemed a Class I recall by the Food & Drug Administration (FDA). A Class I recall indicates a situation in which there is a reasonable probability that use of the product will cause injury or death.
Medtronic said the classification from the FDA does not change the recommendations made to physicians in the January 2008 letter and there is no new action required of physicians or patients in regards to the defective SynchroMed and IsoMed infusion systems.
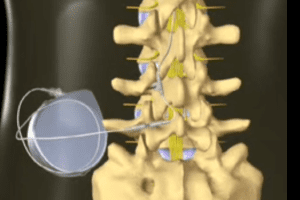
Medtronic’s SynchroMed and IsoMed intrathecal drug delivery systems consist of an implantable pump and catheter that deliver small quantities of drug directly into the intrathecal space in the spine. These devices are used to treat chronic, intractable pain and for the management of severe spasticity of cerebral or spinal origin.
On January 16, Medtronic sent a letter to healthcare providers informing them of an increase in the rate of reported inflammatory mass cases in patients who have received intrathecal drug delivery through its SynchroMed and IsoMed implantable infusion systems.
This letter was an update to two previous communications on this topic that Medtronic issued in 2001 and 2003. The risk of inflammatory mass formation has been included in the labeling for Medtronic’s SynchroMed and IsoMed implantable drug infusion systems as either a Warning or Precaution, since 2001 and in the prescribing information for Infumorph (preservative-free morphine sulfate for microinfusion pumps) since 2003.
Inflammatory mass is a chronic inflammatory or granulomatous mass at or near the tip of intrathecal catheters and has been reported with the infusion of morphine, baclofen and other physician-prescribed drugs and/or mixtures, including pharmacy-compounded drugs. In the January letter, Medtronic noted an increase in reported cases of inflammatory mass associated with intrathecal drug delivery from 0.1 percent reported to date in 2001 to 0.5 percent reported to date in 2007.
The company said that the actual incidence was likely higher than stated due to under-reporting. Medtronic said it has received FDA approval to update the SynchroMed and IsoMed labeling to include this new data.
According to Medtronic’s press release, the most frequently reported symptoms of inflammatory mass are decreased therapeutic response, pain, and neurological deficit/dysfunction. Serious reported symptoms include paralysis and other neurological impairments.
To date, there have been no reported deaths associated with this issue. Medtronic said the development of inflammatory mass was associated with a wide range of doses and concentrations of opioids. The risk of inflammatory mass occurrence also appeared to increase over time and with higher concentrations of opioids
In the January letter, Medtronic cautioned clinicians to utilize the lowest effective dose and concentration of opioids and to monitor patients closely for early clinical symptoms of inflammatory mass. The letter also recommended diagnostic steps for physicians to consider for patients who have new neurological symptoms aimed at preventing more severe outcomes.
Medtronic said patients with questions should talk to their physician or contact Medtronic Patient Services at 1-800-510-6735, Monday – Friday, 8 a.m. to 5 p.m. CDT. Physicians with medical questions related to Medtronic therapies should contact Medtronic at 1-800-328-0810, Monday – Friday, 8 a.m. to 5 p.m. CDT.
The personal injury attorneys at Parker Waichman LLP offer free, no-obligation case evaluations. For more information, fill out our online contact form or call 1-800-YOURLAWYER (1-800-968-7529).


