
Betaseron Side Effects Hepatic Toxicity. The FDA approved Betaseron on July 23, 1993. Betaseron is approved for the treatment of relapsing forms of multiple sclerosis to reduce the frequency of clinical exacerbation’s. On April 22, 2005 healthcare professionals were warned regarding the prescribing information for Betaseron (interferon beta-1b) pertaining to hepatic toxicity. There have been […]
Betaseron Side Effects Hepatic Toxicity. The FDA approved Betaseron on July 23, 1993. Betaseron is approved for the treatment of relapsing forms of multiple sclerosis to reduce the frequency of clinical exacerbation’s. On April 22, 2005 healthcare professionals were warned regarding the prescribing information for Betaseron (interferon beta-1b) pertaining to hepatic toxicity.
There have been reports during post-marketing safety surveillance of serious hepatic injury including autoimmune hepatitis and severe liver damage leading to hepatic failure and transplant. Liver function testing is recommended at regular intervals (one, three, and six months) following introduction to Betaseron therapy, and then periodically thereafter in the absence of clinical symptoms.
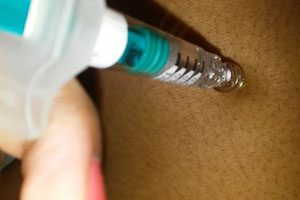
Injection site necrosis , which occurs in about 5% of patients during the first four months of therapy, has been reported in post-marketing studies even after a year of treatment.
During the clinical trial of interferon beta-1b, there were four suicide attempts and one completed suicide among those taking interferon beta-1b. Common side effects include flu-like symptoms (fatigue, chills, fever, muscle aches, and sweating) and injection site reactions (swelling, redness, discoloration, and pain).
The personal injury attorneys at Parker Waichman LLP offer free, no-obligation case evaluations. For more information, fill out our online contact form or call 1-800-YOURLAWYER (1-800-968-7529).
Our lawyers for Betaseron side effects are here to help you when you need it the most.


