
UNITED STATES – According to an online news article published by www.medtechdive.com, and a safety notification published by www.fda.gov, Teleflex Medical recently recalled more than 6 million endotracheal tubes after two deaths were linked to the medical device. Teleflex Medical manufactures endotracheal tubes used in patients who need assistance breathing during medical procedures that require […]
Teleflex Medical issues recall
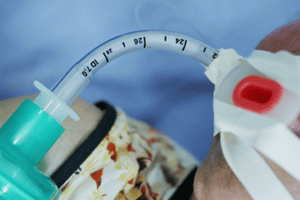
UNITED STATES – According to an online news article published by www.medtechdive.com, and a safety notification published by www.fda.gov, Teleflex Medical recently recalled more than 6 million endotracheal tubes after two deaths were linked to the medical device.
Teleflex Medical manufactures endotracheal tubes used in patients who need assistance breathing during medical procedures that require inhaled anesthetic gases. The endotracheal tubes are intended to open a patient’s airway, provide ventilation and administer anesthesia.
Teleflex Medical (along with the United States Food and Drug Administration (FDA)) has revealed that patient injuries and two deaths have been linked to the failure of the company’s endotracheal tubes. According to Teleflex Medical, the 15-millimeter connector to the endotracheal tubes have become disconnected, stopping the flow of air to patients. The connector can be removed. However, substantial force is required to disconnect the connector.
Teleflex Medical and the FDA received a total of 179 complaints regarding the endotracheal tubes and 192 incidents that involved device disconnection. Of these reported device failures, one patient sustained injuries while two patients died from their injuries. When the endotracheal tubes became disconnected, stopping the flow of air, the medical teams decided to remove the tubes and reintubate them. The two deaths happened during reintubation. Because device failure resulted in injuries and two deaths, the FDA has classified the recall as Class I, the most severe classification possible.
The following devices are included in the recall of more than 6 million endotracheal tubes:
The distribution dates for the affected products are from October 2016 to May 2019.


