
A Food and Drug Administration (FDA) advisory panel has recommended that the label for the Essure birth control device clearly state that Essure is a surgical device and the implant should come with more comprehensive instructions for insertion and follow-up monitoring. After extensive discussion of post-marketing data, peer-reviewed studies, and hours of patient comments about […]
FDA Panel Urges Essure device Label Changes
A Food and Drug Administration (FDA) advisory panel has recommended that the label for the Essure birth control device clearly state that Essure is a surgical device and the implant should come with more comprehensive instructions for insertion and follow-up monitoring.
After extensive discussion of post-marketing data, peer-reviewed studies, and hours of patient comments about the Essure device, the agency’s Obstetrics and Gynecology Devices panel offered suggestions for best practices, stronger patient education, and increased collection of data, Medpage Today reports.
The panel recommended:
Members recommended label changes to indicate that the implantation is a “surgical” rather than “nonsurgical” procedure, as currently stated. The label should also indicate that the procedure may be more dangerous for the small percentage of women whose fallopian tubes are not “straight shots” but “tortuous,” said Dr. Charles Coddington III, a gynecological surgeon at the Mayo Clinic.
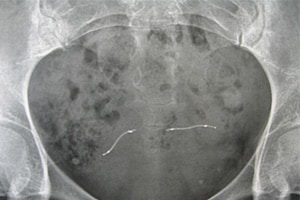
Essure was developed by the California company Conceptus (now a Bayer Health subsidiary), and approved in 2002 as a minimally invasive implant procedure. Nickel titanium coils are inserted into the fallopian tubes to trigger the growth of scar tissue that blocks the tubes and prevents pregnancy. The woman must have a follow up at three months to confirm that the fallopian tubes are blocked. Women are urged not to stop using alternative contraception until the blockage has been confirmed.
As of June 1, 2015, about 5,093 reports of adverse effects in women implanted with Essure had been filed with the FDA.
Dr. Cheryl Iglesia, temporary committee chair and surgeon at MedStar Washington Hospital Center, summarized the panel’s consensus that the device was appropriate for patients who wanted permanent contraception and who had thoroughly discussed the device’s advantages and potential harms with their physician, according to Medpage Today. Patients with chronic pelvic pain, autoimmune disorders, hypersensitivity to nickel or other metals, pelvic inflammatory diseases, or a history of abnormal uterine bleeding, may not be well-suited to this device, Iglesia said.
The committee also recommended better monitoring and earlier removal of the device when it causes problems.
During the public portion of the discussion, more than 30 patients spoke, describing severe abdominal pain, bleeding irregularities, migraines, painful sex, autoimmune disorders, extreme fatigue, psoriasis, perforation of the uterus, and even narcolepsy and ccataplexy—all symptoms they attribute to the implantation of the Essure System. Some women said they became pregnant despite the device, Medpage Today reports.


