
The U.S. Food and Drug Administration (FDA) has issued its strongest possible warning about the use of codeine to relieve children’s pain after surgery to remove their tonsils or adenoids. The FDA reports that deaths have occurred after surgery in children with obstructive sleep apnea who received codeine for pain relief following such surgeries, according […]
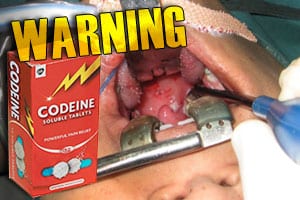 The U.S. Food and Drug Administration (FDA) has issued its strongest possible warning about the use of codeine to relieve children’s pain after surgery to remove their tonsils or adenoids.
The U.S. Food and Drug Administration (FDA) has issued its strongest possible warning about the use of codeine to relieve children’s pain after surgery to remove their tonsils or adenoids.
The FDA reports that deaths have occurred after surgery in children with obstructive sleep apnea who received codeine for pain relief following such surgeries, according to Reuters. These patients may already have underlying breathing problems that made them particularly sensitive to the breathing difficulties that can result when codeine is converted to morphine by the liver. Some children, the FDA said, are “ultra-rapid metabolizers” of codeine, meaning that their liver converts codeine to morphine in higher than normal amounts. “High levels of morphine can result in breathing difficulty, which may be fatal,” the FDA warns.
Following a review of deaths and serious side effects in children, the FDA is requiring a new boxed warning–the agency’s strongest warning–to be added to the label of codeine-containing products. The labels will also include a recommendation that the drugs not be used in these patients in this setting, Reuters said.
The FDA has asked health care professionals to use an alternate pain reliever after tonsil or adenoid surgery. In addition, the agency said, parents and caregivers need to be aware of the risks of codeine treatment and they should ask for a different pain medicine if their child is prescribed codeine following surgery.


