
Last week the Food and Drug Administration (FDA) responded to a petition demanding removal of Essure permanent birth control device from the market. The petition was signed by more than 2,100 women who are concerned about injuries and adverse effects they say are caused by Essure. The FDA forwarded the petition to the Office of […]
FDA Issues Response to Petition for Removal of Essure Birth Control from Market
Last week the Food and Drug Administration (FDA) responded to a petition demanding removal of Essure permanent birth control device from the market. The petition was signed by more than 2,100 women who are concerned about injuries and adverse effects they say are caused by Essure.
The FDA forwarded the petition to the Office of Compliance, which will investigate the claims about the device and “pursue actions as deemed necessary,” Arizona television station ABC15 reported.
The citizens’ petition was submitted to the FDA in January by a Florida law firm. Famed consumer activist Erin Brokovich assisted the group in collecting signatures. Women in the group requested Brokovich’s help in bringing attention to injuries and adverse effects they attributed to Essure. Brockovich’s web site has women’s accounts of chronic pain, colon perforation caused by movement of the device, debilitating headaches, implants that were no longer visible on scans, and pregnancies that occurred despite the presence of the device.
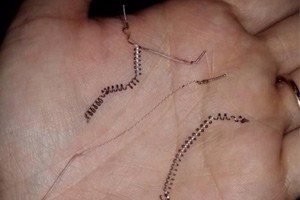
The FDA considers Essure a permanent form of birth control. Essure consists of flexible metal coils that are implanted in the woman’s fallopian tubes. Scar tissue that forms around the coils prevents fertilization. According to Bayer HealthCare Pharmaceuticals’ online materials, the implant procedure can be done on an outpatient basis in a doctor’s office and takes about 10 minutes to perform.
Essure received FDA premarket approval in 2002 and such approval normally shields the manufacturer from product liability claims. But lawsuits filed by women who claim injuries from Essure say the manufacturer violated the conditions for that approval, thereby making the Conditional Premarket Approval “invalid” and the product “adulterated” under FDA definition. The citizens’ petition makes the same claims and the petitioners requested the FDA take a number of actions against Bayer HealthCare Pharmaceuticals, including a recall of the device, according to ABC15.
The petition claims the original Essure manufacturer, Conceptus, Inc., perpetrated fraud during the clinical trials, violated the terms of the FDA’s premarket approval of the device, ABC15 reports. The company is also accused with violating federal laws in the manufacturing and marketing of Essure. Bayer HealthCare bought Conceptus in 2013.
Doctors and patients have filed more than 4,500 adverse event reports with the FDA, describing side effects and injuries they allege were caused by Essure. Reports include deaths of women implanted with the device and premature births in women who became pregnant while implanted with Essure, according to ABC15.
Bayer HealthCare Pharmaceuticals said it “stands behind the safety and efficacy of Essure,” but will respond “to any questions that may have related to the petition.” The company said it would “aggressively” defend itself in the product liability lawsuits, according to ABC15.
Read more at: DEFECTIVE MEDICAL DEVICE LAWYERS


