
The U.S. Food and Drug Administration (FDA) has issued a safety alert warning against the off-label use of the hyaluronic acid product Expression injectable, manufactured by Enhancement Medical LLC. According to MedWatch, Expression injectable is approved as an intranasal splint. It is used to reduce the amount of bleeding and swelling and reduce the risk of adhesions […]
 The U.S. Food and Drug Administration (FDA) has issued a safety alert warning against the off-label use of the hyaluronic acid product Expression injectable, manufactured by Enhancement Medical LLC.
The U.S. Food and Drug Administration (FDA) has issued a safety alert warning against the off-label use of the hyaluronic acid product Expression injectable, manufactured by Enhancement Medical LLC.
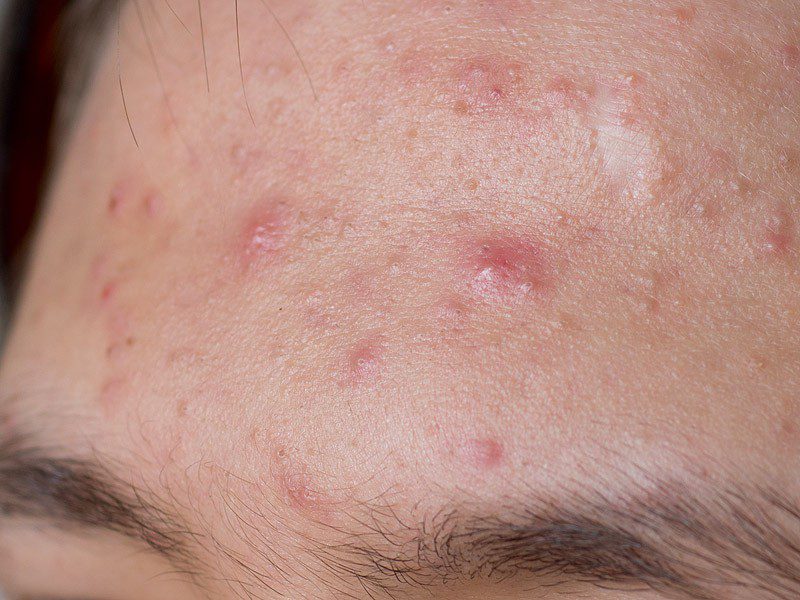
According to MedWatch, Expression injectable is approved as an intranasal splint. It is used to reduce the amount of bleeding and swelling and reduce the risk of adhesions between the septum and nasal cavity after surgery or trauma. The FDA has not approved this product as a dermal filler. Using a medical product in a manner not approved by the FDA constitutes as “off-label” use.
Using the injectable to fill in wrinkles has been associated with a number of side effects, the FDA reports. These adverse events include swelling, tenderness, firmness, lumps, bumps, bruising, pain, redness, discoloration, itching, and the development of hard nodules. One patient was left with “obvious deformity” after having the product injected in the face; firm masses developed and the attempt to remove them was unsuccessful.
The agency says that patients who have received expression as a dermal filler “should be monitored for adverse events and referred for corrective treatment when appropriate,”
Enhancement Medical LLC received a June 4th warning letter from the FDA citing “multiple quality system, correction/removal, and medical device reporting violations that were revealed during an inspection.” Among other things, the letter states that the product was inappropriately touted as an “injectable filler”.
The FDA notes that some of the ingredients in Expression injectable are similar to FDA-approved wrinkle fillers, but it has not been approved for this use.


