
The U.S. Food and Drug Administration (FDA) permitted sale of a device—the BSD-2000—which is used to combat cervical cancer in women who are too ill to undergo chemotherapy. At issues is that the device has not been fully tested. The BSD-2000 uses intense heat to kill off cancer cells and is only used in very […]
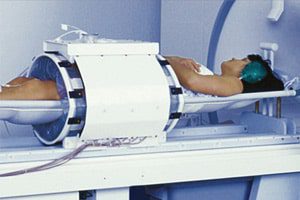 The U.S. Food and Drug Administration (FDA) permitted sale of a device—the BSD-2000—which is used to combat cervical cancer in women who are too ill to undergo chemotherapy. At issues is that the device has not been fully tested.
The U.S. Food and Drug Administration (FDA) permitted sale of a device—the BSD-2000—which is used to combat cervical cancer in women who are too ill to undergo chemotherapy. At issues is that the device has not been fully tested.
The BSD-2000 uses intense heat to kill off cancer cells and is only used in very specific circumstances when women are extremely ill from cervical cancer, according to The New York Times. In the two years since the device was sold, those few hospitals that purchased the BSD-2000, a $500,000 device, have not participated in a patient study that the manufacturer agreed to perform as part of the device’s approval. In fact, cancer experts have said they were surprised the regulators cleared the BSD-200 to begin with.
The FDA approved the treatment under the humanitarian device exemption regulation, a program that is based on what The New York Times described as “good intentions.” In this case, for women who are suffering from advanced cervical cancer, a small group of patients. “I see, like, one patient like this a year,” Dr. Junzo P. Chino, a cancer expert at Duke University, told The New York Times.
In the case of these types of approvals, there is really no incentive for expensive clinical trials for products that are used for a very small demographic. The manufacturer only needs to show that the device is safe and provides “probable” benefits. Normal standards mandate that the device maker prove efficacy, according to The New York Times.
Critics argue that ineffective products are approved under the exemption, selling for years while potentially effective and life-saving devices may never receive approval, according to The New York Times. Doctors may also use the device in whatever way they choose, so-called “off-label” uses, which has been criticized, as well.
Since the BSD-2000 received exemption in 2011, the for-profit Cancer Treatment Centers of America purchased two of the devices. Meanwhile, the company would not answer written questions about how many cancer patients were treated with the devices, the types of cancers that were treated, and what patients were charged for treatments, according to The New York Times. “The provision is a good one, but the structure to get good effectiveness data is weak,” said Larry Kessler, a former FDA official and current professor at the University of Washington in Seattle.
The BSD-2000 utilizes microwave energy that increases the body’s temperature to as much as 113 degrees Fahrenheit (45 Celsius), according to The New York Times. The treatment is known as hyperthermia and is based on the notion that these high temperatures can cause damage to, and kill, the cancer cells and with minimal damage to healthy tissue.
Research has also found that increased cell temperatures also increase radiation and chemotherapy efficacy; hyperthermia is generally used in collaboration with these treatments, The New Yorks Times reported.
BSD Medical makes another hyperthermia machine, the BSD-500, which received FDA approval in 1983 for the treatment of breast cancer and tumors located just below the skin. Studies note the potential efficacy of the BSD-2000 device, which was developed to treat so-called “deep-seated” tumors, which are often seen in the pelvis. The device was unable to achieve medical acceptance for about 30 years.
Dr. Elizabeth Repasky, a hyperthermia expert at the Roswell Park Cancer Institute in Buffalo, told The New York Times” that she is concerned that agency’ choice to approve the BSD-2000 with limited data collection could adversely impact technology. “It is not helpful to hyperthermia to have yet another uncontrolled study,” Dr. Repasky noted.


