
Two recent actions involving more than 110,000 components of Intuitive Surgical’s da Vinci surgical robot system have been deemed Class II recalls by the U.S. Food and Drug Administration (FDA). The FDA’s Class II designation involves situations in which “use of or exposure to a violative product may cause temporary or medically reversible adverse health […]
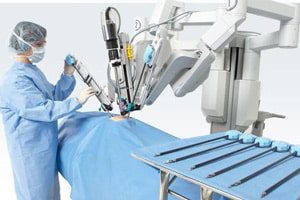 Two recent actions involving more than 110,000 components of Intuitive Surgical’s da Vinci surgical robot system have been deemed Class II recalls by the U.S. Food and Drug Administration (FDA).
Two recent actions involving more than 110,000 components of Intuitive Surgical’s da Vinci surgical robot system have been deemed Class II recalls by the U.S. Food and Drug Administration (FDA).
The FDA’s Class II designation involves situations in which “use of or exposure to a violative product may cause temporary or medically reversible adverse health consequences.”
On November 15th, Intuitive notified customers that a component in the da Vinci’s Endowrist instrument could detach, MassDevice.com reports. The company said the problem affects devices made before October 2011 and involves the jaw insert in the da Vinci’s large needle drivers and mega needle, according to a company press release.
A surgeon should notice if the component detached during surgery, Intuitive’s statement indicated; however, Intuitive’s senior vice president of scientific affairs, David Rosa, told MassDevice.com that at least one event involving this defect resulted in a patient having to undergo a second surgery to remove a piece of the da Vinci. About 1,000 products were affected, Rosa noted.
The second recent Class II designation for the da Vinci system was announced on December 3rd following a November 11, 2013 “Urgent Medical Device Recall” letter issued by Intuitive. The matter involved an instrument arm that could stall during procedures, according to both Intuitive Surgical and the FDA. That recall involved 1,386 da Vinci device arms, MassDevice.com reported.
These recalls are the latest setbacks for Intuitive, which has experienced a dramatic spike in adverse events reported to the FDA, according to MassDevice.com. Intuitive also faces mounting litigation over alleged injury, complication, and death reports involving its da Vinci robot.
In 2004, approximately 33.3 injury reports per 100,000 procedures were received, according to The Wall Street Journal. The figure rose to 50 reports per 100,000 procedures in 2012 and, of the 282 reports received, 28 involved deaths, up 34 percent from 2011. A draft analysis of adverse event reports co-authored by doctors at Rush University Medical Center, the University of Illinois, and the Massachusetts Institute of Technology, revealed a significant increase in injury and death rates tied to robotic surgery, as well.


