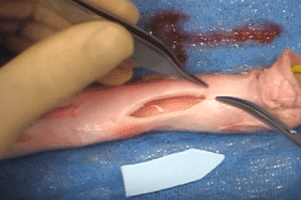
Recalls of CMF Distraction System Jaw-Stabilizing Device Handed Two Class I Designations
Deaths Involved DePuy Synthes CMF. Parker Waichman LLP is reviewing claims concerning serious injuries and deaths involving the DePuy Synthes Craniomaxillofacial (CMF) Distraction System Jaw-Stabilizing device. DePuy Synthes is [...]