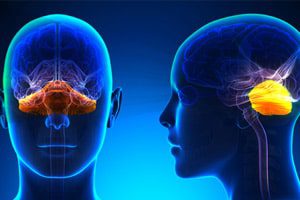
Cerebellar Atrophy Reported in Dilantin Patients
The U.S. Food and Drug Administration (FDA) has approved labeling changes stating that cerebellar atrophy has been reported in patients taking phenytoin. This nervous system adverse reaction appears to occur [...]