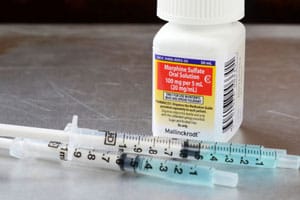
Bryant Ranch Prepack Morphine Sulfate Extended-Release Tablets Lawsuits
On June 29, 2022, the U.S. Food and Drug Administration announced that Bryant Ranch Prepack Inc. had issued a recall concerning its Morphine Sulfate 60 mg Extended-Release tablets and Morphine [...]
