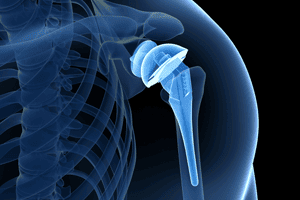
Zimmer Biomet Comprehensive Reverse Shoulder Recall
The Food & Drug Administration has issued a Class 1 recall on the Zimmer Biomet Comprehensive Reverse Shoulder. The FDA has noted that the device has a high fracture rate. [...]