
If you are inquiring about the November 2023 recalled eye drops click here. Bausch & Lomb Lens Solution Fungus Outbreak.Although Bausch & Lomb and the FDA have not ordered a recall of any contact lens solutions other than ReNu With MoistureLoc, the continued spread of Fusarium keratitis cases around the globe raises serious questions as to […]
If you are inquiring about the November 2023 recalled eye drops click here.
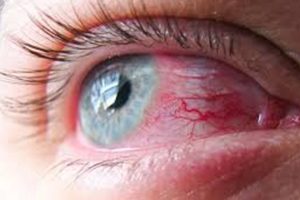
Bausch & Lomb Lens Solution Fungus Outbreak.Although Bausch & Lomb and the FDA have not ordered a recall of any contact lens solutions other than ReNu With MoistureLoc, the continued spread of Fusarium keratitis cases around the globe raises serious questions as to the safety of these products in general.
Since the fungal infection at the center of the current problem is relatively rare, experts are troubled by spikes in its occurrence in Asia (Singapore and Hong Kong), the United States, and Europe all at the same time.
While many of the infections have not been linked to any contact lens product, the fact that such a high percentage of the reported cases have appeared in contact lens wearers who used one or more of the solutions is a serious concern.
Bausch & Lomb and the FDA are still entertaining the possibility that the cases wherein a product other than ReNu With MoistureLoc was used may simply be within the “normal” range of Fusarium infections and thus, not related to any contact lens solution. While this theory has surface appeal and seems logical when individual cases and products are considered, many are not so sure it applies to the current problem.
The appearance of so many cases of a “rare” condition at approximately the same time on a global scale suggests to some experts that a broader problem may exist with the anti-fungal properties of the current crop of contact lens cleaning solutions and the scaling back of preventive cleaning practices by contact lens wearers in general.
From the very beginning of the involvement of the FDA and CDC in the investigations, the FDA warning has been a serious one. “This is to inform you of a recent increase in the number of reports in the United States of a rare but serious fungal infection of the eye in soft contact lens wearers. The infection, a fungal keratitis caused by the Fusarium fungus, may cause vision loss requiring corneal transplants.
“Both the Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) are investigating this situation. At this time, Bausch and Lomb has agreed to stop shipping the ReNu MoistureLoc brand contact lens solution. This Notification will be updated as more information becomes available.”
The FDA and CDC have been just as concerned, however, with stressing the need for contact lens wearers to follow a combination of procedures designed to ensure the highest success rate for disinfecting contact lenses. These include:
In addition, “regardless of which cleaning/disinfecting solution used, wearers may want to consider performing a ’rub and rinse’ lens cleaning method, rather than a no rub method, in order to minimize the number of germs and reduce the chances of infection.”
The FDA also provided specific “Advice to Patients” on this topic, which can be found at http://www.fda.gov/cdrh/medicaldevicesafety/atp/041006-keratitis.html.
Thus, the unusually high number of Fusarium cases in contact lens wearers using several current cleaning solutions may be a combination of several factors rather than simply one product being at fault or susceptible to fungal contamination.
While ReNu With MoistureLoc seems to be associated with an inordinately large number of cases of Fusarium keratitis, the potential link to current cleaning solutions in general cannot be disregarded.
Bausch & Lomb’s ReNu MultiPlus solution was used by at least 15 of those people and unspecified ReNu solutions were used by seven more. Solutions made by Alcon and Advanced Medical Optics (AMO), although in no way linked specifically to the fungal infection yet, were reportedly used by three patients each. Some of the infected people may have used more than one product.
As a result of so many cases of this rare infection occurring in contact lens wearers who also use a number of currently available cleaning solutions, the fact that one or more of the products is not specifically associated with a “disproportionately” high number of cases may not be a reliable indicator that only ReNu With MoistureLoc is a problem.
Clearly, Bausch & Lomb’s products must be considered suspect now that they have been linked to infected users on three continents but the company’s problems go much deeper than that since there is the continuing perception that it has been less than forthcoming about its knowledge of the problem and has not reacted in a way that demonstrates concern for the public’s wellbeing.
Allegations and eye wash lawsuits now abound that claim that Bausch & Lomb has issued contradictory statements and has done far too little to isolate the problem, advise the public, recall the product, and investigate the source of the fungus.
As we previously reported, even last week, no sooner had the ink dried on a Wall Street Journal report that Bausch & Lomb had learned of eye infections linked to its ReNu With Moisture-Loc from Hong Kong health officials in November 2005, than the timeline was moved backward.
While Bausch & Lomb claimed that the November time frame was the very earliest it knew of a potential problem, it appears that, for a second (or even third) time, the company may have been guilty of two of the cardinal sins of product recalls; delay and misinformation by the manufacturer.
Bausch & Lomb first stated February 18, 2006 was the earliest indication that there may be “an unusual occurrence with this infection.” That date, however, was somewhat misleading, since Hong Kong health officials had admittedly told the company in November 2005, that they had “noted an increase in hospital admissions due to contact-lens-related keratitis from June to September 2005.”
The company claimed that nothing in the November contact with Hong Kong authorities, or the investigation being done at the time, seemed to require further action since no definitive link had been made between its product and the “spike” in Fusarium infections.
Apparently, however, the Hong Kong government “alerted Bausch & Lomb Inc. to eye infections in users of its contact lens solution in September 2005, way before the company withdrew its product in February this year.” (Reuters 4.27.06)
According to that report, Hong Kong’s Health Department had detected the spike in Fusarium cases in July and August and, as a result, had conducted tests of the eye solution.
Although no link had yet been made in September, this latest revelation clearly indicated to experts that there was certainly a reason for Bausch & Lomb to take more than a passive role where a very serious eye-related problem was detected in users of one of its eye-care-related products.
Although Bausch & Lomb reported the Hong Kong “incident” to the FDA in December 2005, its report stated that “no causal factors can be determined and no conclusion can be drawn.”
That optimistic appraisal of the situation would not have sounded a warning in the U.S. It also may have lulled Bausch & Lomb into a false sense of security regarding the safety of its product thereby delaying its response to the problem when it finally burst onto the scene in April.
In any event, the shifting timeline has not been a good thing for Bausch & Lomb. Crisis management experts and business analysts have pointed out that the delay in implementing a crisis management plan was inexcusable and therefore highly damaging to the company’s overall image, stock value, and sales of other unrelated products.
Those observations have become even more applicable now that the company’s stock price has fallen considerably and another of its leading products may have a similar infection-related problem. Bausch & Lomb’s effort to keep ReNu MultiPlus on the market while minimizing the risk to the public that this second product might be causing cannot be helping the company’s image either. The apparent spread of the outbreak to Europe is also quite problematic for the company as the crisis it faces has truly become one of global proportions.
The personal injury attorneys at Parker Waichman LLP offer free, no-obligation case evaluations. For more information, fill out our online contact form or call 1-800-YOURLAWYER (1-800-968-7529).


