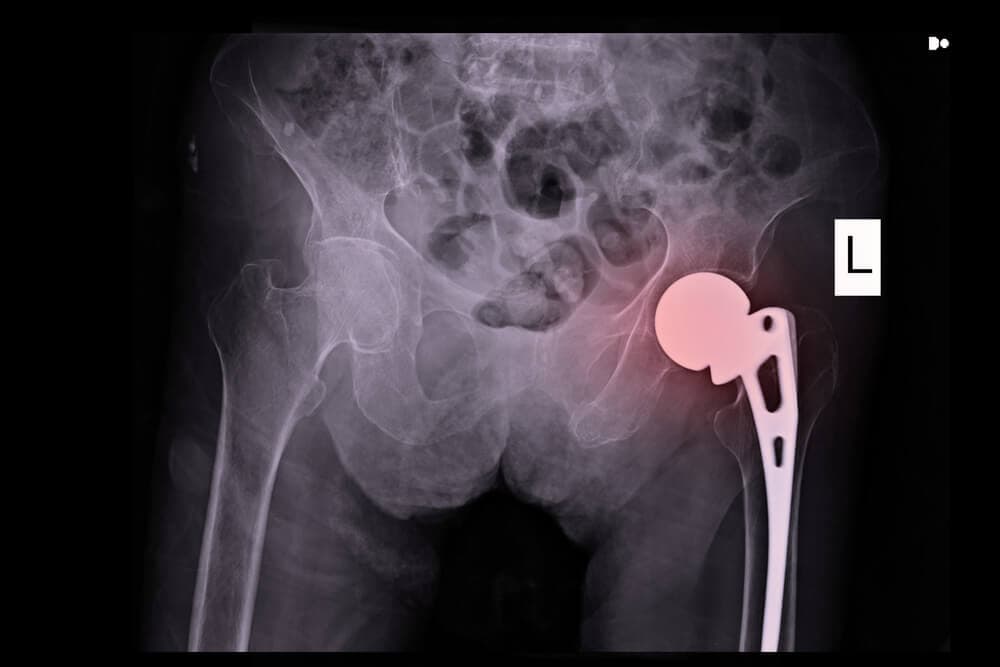
Stryker LFIT V40 Femoral Head Recalled
Stryker continues to face growing metal-on-metal hip implant litigation as lawsuits mount over the company’s LFIT V40 Femoral Head. The hip replacement component was recalled in 2016 due to reports [...]