Defective Medical Device Lawyers Fighting For Your Health And Safety
More than a decade ago, the defective medical device lawyers at Parker Waichman LLP called for increased governmental oversight of medical devices and diagnostic machines. We observed that numerous medical devices, such as two different versions of Stryker’s hip implants, heart defibrillators manufactured by Medtronic, and certain Composix Kugel Mesh patches, manufactured by C.R. Bard subsidiary Davol Inc., had caused thousands of people to suffer serious and even life-threatening injuries.
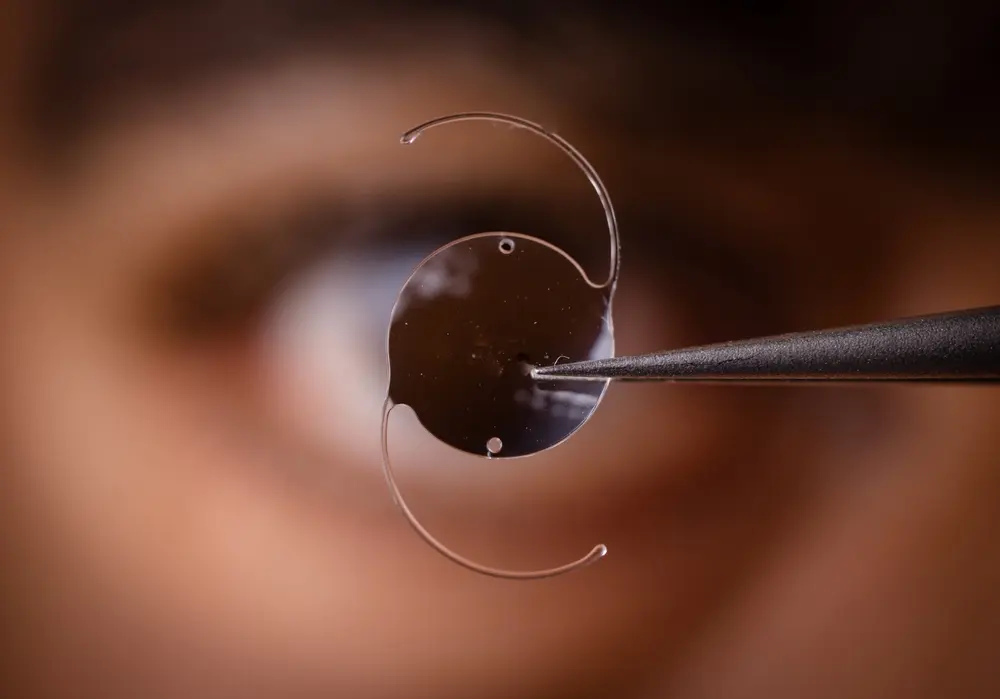
Since then, many other medical devices and treatments have failed, causing numerous people to suffer. Medical devices such as DePuy’s metal-on-metal hip implants, Bayer’s Essure birth control device, and Medtronic’s Infusion insulin pump have been linked to severe complications. Many of such devices have been recalled by the U.S. Food and Drug Administration (FDA).
Today, new FDA regulations should help to address these issues, but that won’t protect those who have already suffered injuries due to defective medical devices. If you have been harmed by a device that was supposed to help improve your health, call us. We can provide a free case evaluation to determine if you should pursue a legal claim for compensation.
The Call For A More Rigorous Regulatory Scheme
In 2008, researchers from the University of California at San Francisco argued that medical devices did not have sufficiently stringent regulations to guarantee patient safety. These researchers found that new medications went through more rigorous testing and analysis before receiving FDA approval when contrasted with medical devices. (For clarity, a medical device is an implement that can be implanted, such as a knee or hip, or a machine that somehow assists the body to perform its natural functions.)
Medical device manufacturers can rely on expedited approval for their latest device when the item functions similarly to a device previously approved by the FDA. The FDA dispenses with rigorous review in these cases and permits the items to go to market. The researchers proposed that the FDA approval should be the first step toward the lawful use of medical devices, not the last step.
The next step, they suggested, should be a review of the device’s technology by an independent body of researchers and scientists. Further evaluation after the FDA’s initial approval would allow physicians to learn about potential limitations of the device and have a thorough understanding of the possible side effects from use of the device. It would also determine how long the device will last before replacement is required.
The FDA has been working on changes to its processes in recent years, but it still has a ways to go to update how it approves these potentially life-saving or life-destroying devices.
Fighting for Medical Device Victims
Parker Waichman LLP has offices nationwide, making it easy to access top-tier legal help no matter where you are. Our local teams are familiar with regional laws and courts, giving you a strategic advantage.
Ready to discuss your product liability case? Contact our nearest office today for a free consultation. Call now (516) 466-6500 or schedule an appointment online for a free consultation.