How to Receive the Compensation You Deserve frin ab Exactech Optetrak Total Knee Lawsuit Settlement

Exactech, Inc., the medical device maker has recalled over 300,000 knee, ankle, and hip implant systems. According to the U.S. Food & Drug Administration (FDA), Exactech notified doctors about a problem with its knee replacement and ankle replacement systems in August 2021. In February 2022, the company expanded the Exactech recall and promised to notify patients. This notification is critical, as most knee and ankle implant patients do not know what device was implanted. In April 2022, Exactech issued an Urgent Medical Device Correction, the most serious form of recall. The company also created a letter to patients and posted it to their website – but as of June 2022, it appears that few patients have actually received recall letters. Contact us to learn more about the Exactech knee replacement lawsuit.
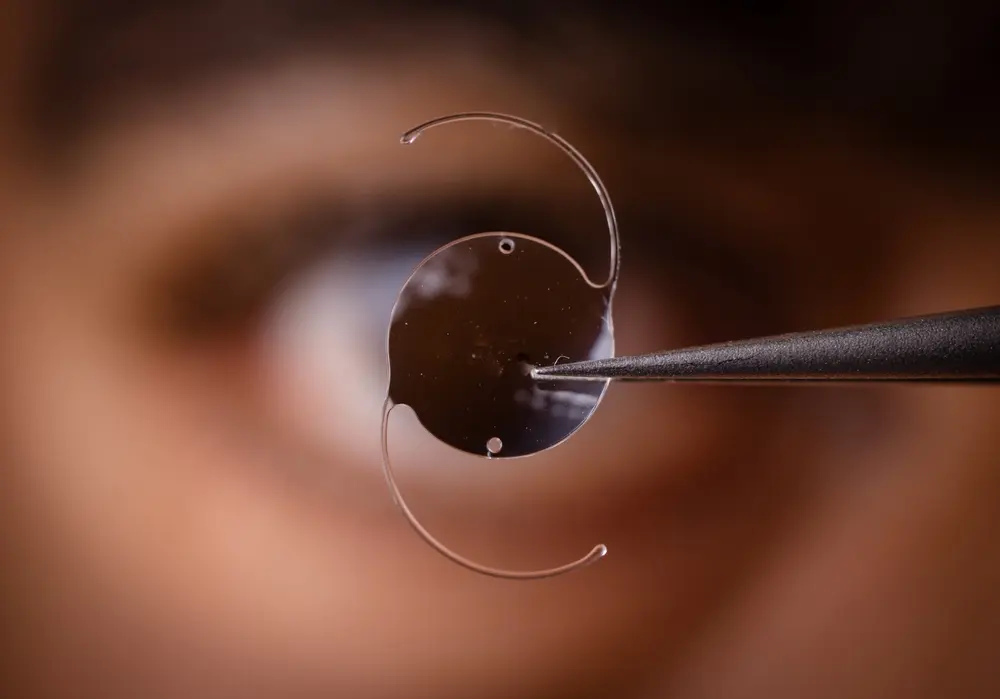
According to the company’s recall announcement, the Exactech recall was necessary due to defective polyethylene (plastic) inserts. As a result of the defects, the polyethylene inserts degrade quickly, and patients with the affected implants may require revision surgery to correct the issue. In some cases, patients suffer bone loss, which can require revision surgery.
Exactech’s recall affects the following knee and ankle implants that have been manufactured since 2004:
- OPTETRAK®
- OPTETRAK Logic®
- TRULIANT®
- VANTAGE®
- CONNEXION GXL®(hip implants)
- ACUMATCH®(hip implants)
- MCS®(hip implants), and
- NOVATION®(hip implants)
If you or a member of your family have been implanted with any of these knee, hip, or ankle implants, there is a significant chance that your implant is defective.
What is the Problem with the Exactech Recall Knee, Ankle and Hip Implants?
According to Exactech’s recall announcement, the affected implants were packaged in “non-conforming” pre-surgical packaging. The vacuum-sealed packaging failed to protect the knee and ankle implants from oxygen leading to oxidation which can cause bone loss, accelerated production of wear debris, and/or component fatigue fracturing/cracking. In other words, oxidation causes the device to break down prematurely. Moreover, the defective packaging did not contain a second barrier with ethylene-vinyl alcohol to prevent oxygen contamination of the implants prior to implantation into “partial” and “total” knee and ankle replacement patients.
The potentially damaged tibial polyethylene (plastic) insert fits between the tibial component and the femoral component inside the knee implant. Patients who are affected by an Exactech knee recall may have already received a recall letter explaining the issue and what patients should do if they suffer symptoms of a failing knee or ankle implant. Patients who are suffering the following side effects, symptoms, or signs might need a medical assessment and medical attention. The symptoms of knee implant premature wear and/or failure include:
- Unusual and persistent pain,
- swelling,
- redness,
- stiffness of the knee, and/or instability.
Patients who are not suffering the aforementioned symptoms should discuss the recall with their doctor at the next regularly scheduled implant checkups. An x-ray of the implant should be taken and reviewed by your doctor, even if there are no symptoms of a failing implant.
The FDA Issues New Recommendations for Exactech Joint Replacement Patients
Update – March 23, 2023
The FDA is issuing a reminder to patients and healthcare providers about Exactech joint replacement devices manufactured by Exactech from 2004 until August 2021, and the implants were recalled in 2021 and 2022. Numerous Exactech joint replacement devices (including knees, ankles, and hips) were packaged in defective bags, missing an oxygen barrier layer that protects the devices from oxidation. Oxidation can result in accelerated device wear/failure, component cracking or fracture, and the need for corrective revision surgery. Some recalled devices had been linked to a heightened risk of revision surgeries and bone loss due to excessive device wear/failure.
Recommendations for Exactech Joint Replacement Patients
If your joint replacement device is functioning properly and you have no symptoms or pain, the FDA does not advise surgery to remove well-functioning Exactech devices. Contact your physician if you have any Exactech device implanted and experience new or worsening swelling or pain, grinding or other noise, inability to bear weight, or weakness around the implant. If you have an ankle or knee replacement device, you can search Exactech’s online database (https://recall.exac.com) to see if your implant is part of their recall. You will need to submit your device’s serial number.
Recommendations for Health Care Providers
Do not implant any recalled knee, ankle, and hip devices from Exactech. Based on available information, the FDA does not recommend removing well-functioning Exactech joint replacement devices from patients without new or worsening pain or symptoms. Monitor patients with implanted devices produced by Exactech from 2004 through August 2021 for potential device wear, failure, or bone loss. Consider X-rays to further evaluate a patient and their implanted device if you suspect a failure. Discuss potential revision surgery with patients who have worsening pain or joint weakness potentially due to the device on a case-by-case basis. As part of shared decision-making, discuss the risks and benefits of all relevant treatment options. Remove all recalled implant devices from inventory and return them to Exactech. For additional information, visit the Exactech Recall website.
Recalled Device Descriptions
Exactech joint replacement implant devices are designed to be used to replace painful, arthritic joints caused by various conditions in adults, as well as to improve previously failed joint replacement devices where adequate levels of bone and soft tissue are present. All Exactech devices contain a plastic component that should be packaged with multiple oxygen barrier layers as specified in the package specification. The devices were recalled after it was discovered that the sterile packaging was missing the oxygen barrier layers in some lots. The oxygen barrier protects the implant devices from oxidation.
The recalled bags allow oxygen from the air to contact the plastic (polyethylene) component prior to implantation, potentially leading to oxidation of the plastic component over time and increasing the chances of early and excessive implant device wear, device failure, component fracture, new or worsening pain, swelling in the affected area, more bone loss, or the need for revision surgery.
Knee and Ankle Replacement Devices with Defective Packaging
On February 7, 2022, Exactech expanded a voluntary recall on all Optetrak, Logic, and Truliant knee replacements and Vantage total ankle replacements packaged in defective bags, regardless of a device’s label or shelf life. About 80% of knee and ankle replacement devices manufactured since 2004 were packaged in defective bags, which can lead to oxidation over time and the potential risks listed above.
On April 7, 2022, the company issued an updated Urgent Medical Device Correction notice with additional identified devices, advising surgeons, hospitals, and healthcare providers not to implant devices packaged in defective bags. Devices in defective bags were manufactured between 2004 and August 2021 and possibly implanted through February 7, 2022. The FDA classified these as a Class II recall on October 4, 2021.
Hip Replacement Devices with Defective Packaging
In June 2021, Exactech recalled some GXL Liners for Novation, Acumatch, and MCS hip replacement devices due to excessive and premature wear, but the root cause was unknown. The FDA classified these as a Class II recall on July 22, 2021.
In August 2022, Exactech expanded the hip replacement device recall to include all hip devices with polyethylene components packaged in defective bags. The FDA classified these as a Class II recall on September 9, 2022.
The FDA is collaborating with Exactech to assess whether any other joint implants containing polyethylene components packaged in defective bags may result in increased oxidation and similar risks.
The FDA’s Actions Concerning These Recalls
The FDA will continue working with Exactech to evaluate the risks of all joint devices and review information regarding the safety and effectiveness of their joint replacement devices. The FDA is also collaborating with international regulatory agencies to review additional, relevant data concerning the affected devices from registries. The FDA will keep patients, and healthcare providers informed as significant information becomes available.
How to Get Legal Help to Recover Damages for the Exactech Knee Replacement Lawsuit
Patients and surgeons place an enormous amount of trust in surgical implant manufacturers to create safe, effective devices. Patients suffer tremendously when an implant maker does not ensure their products are safe. Patients who were implanted with an Exactech total knee or ankle implant should speak with their doctor to find out if their specific implant is part of this recall. If so, your doctor will determine if you will need revision surgery.
Patients who have been implanted with an Exactech recall implant should speak with our product liability lawyers to find out if they can hold Exactech liable through an Exactech knee implant lawsuit. Surgical implant manufacturers should be held responsible for the injuries their products cause. Our experienced product liability lawyers can help you understand your legal rights and help you bring an Exactech lawsuit.
Why Should You Choose Parker Waichman LLP?
At Parker Waichman LLP, our Knee Implant Lawsuit Lawyers have substantial experience representing clients in defective product cases, including defective medical implant claims, and you can be certain that our firm will work hard to obtain the best possible results in your implant defect case. An implant failure should not be taken lightly, so take the actions today to receive the financial compensation you need and deserve by calling 800-968-7529 to discuss your case.
At Parker Waichman LLP, our product liability lawyers are passionate and dedicated to advocating for the legal rights of every client. Our firm has recovered over $2 billion on behalf of our clients through hard work and determination. Parker Waichman LLP has also received several honors and accolades, including:
- An AVVO rating of 9.8 out of 10
- A listing in Best Lawyers, which is based on extensive peer review
- A peer review rating of “AV Preeminent,” which is the highest rating, from Martindale-Hubbell
- Law dragon’s highest ranking of “5 Dragons.”
If a defective total knee implant has marked you or a loved one, choose Parker Waichman LLP to represent you. We will take the time to listen, conduct a comprehensive investigation into the facts of your case, and make every effort to obtain successful results in your case.
Get Legal Help with Your Exactech Optetrak Total Knee Lawsuit
If you or a family member have suffered harm due to a defective Exactech Optetrak Total Knee Replacement System, as described in the Exactech recall, contact the experienced product liability lawsuit lawyers at Parker Waichman LLP. Our legal professionals are here to help. Contact our defective medical device lawyers for your free consultation by chatting with our online chat, filling out our online contact form, or by calling 800-968-7529.