Understanding the Bausch & Lomb enVista Recall

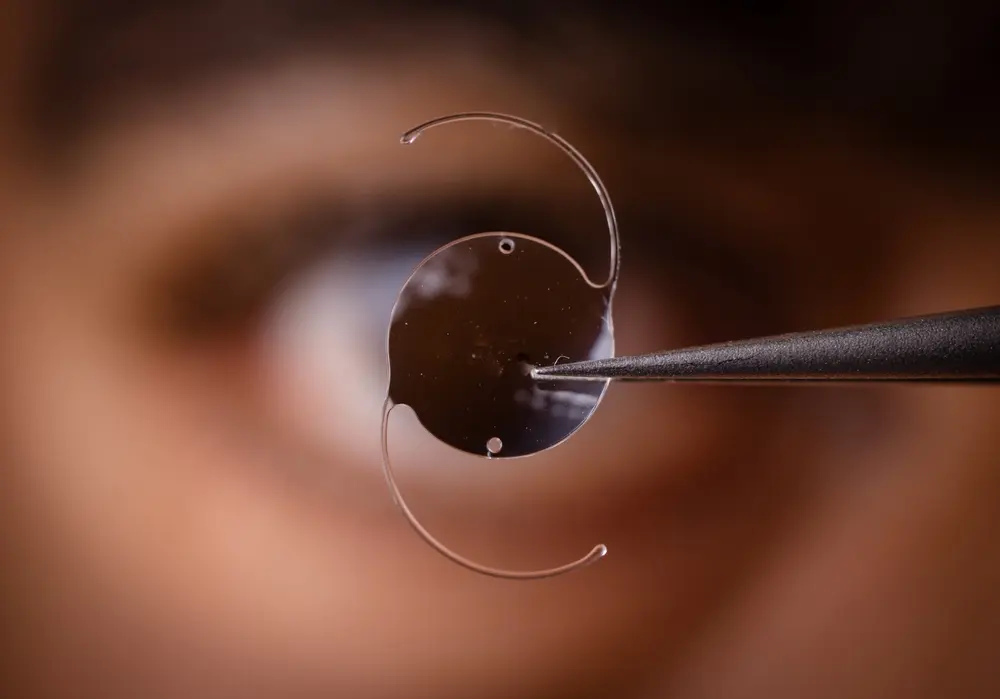
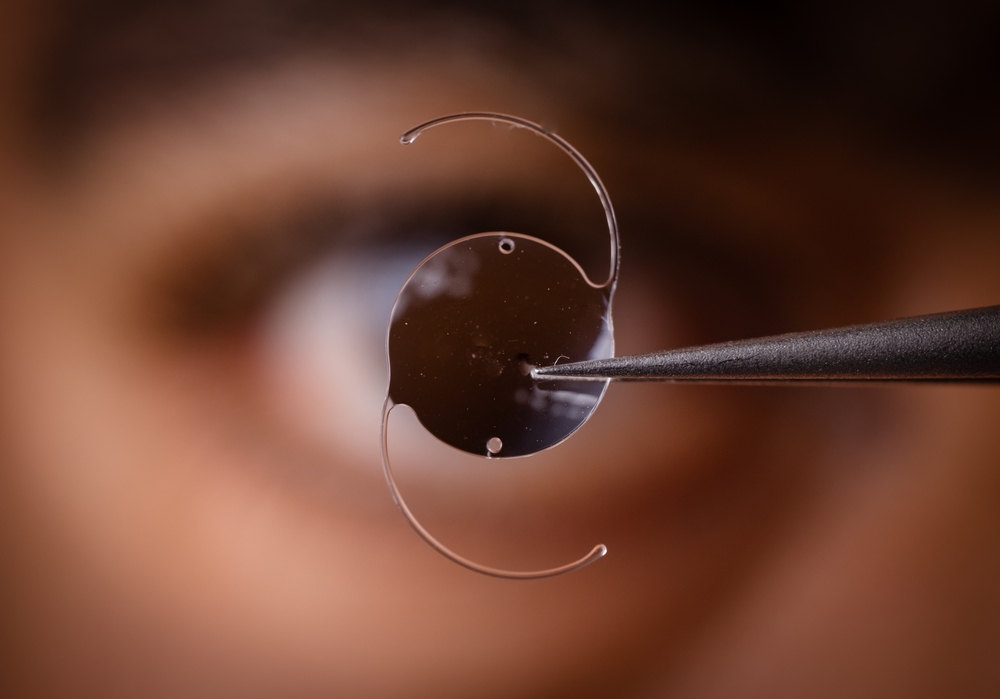
On April 3, 2025, Bausch & Lomb issued a voluntary recall of its popular enVista intraocular lenses (IOLs) in the United States after receiving a surge of reports linking the devices to a dangerous eye condition known as Toxic Anterior Segment Syndrome (TASS). These lenses, which included the enVista Monofocal, Aspire, Envy, and Toric models, had been widely used in cataract and lens replacement surgeries between August 2024 and April 2025.
For patients who trusted these devices to restore or improve their vision, the recall announcement raised serious concerns. Many had already undergone surgery and soon began experiencing severe complications that could threaten long-term eye health. TASS is not just a short-term setback—it can lead to significant pain, inflammation, permanent corneal damage, and even loss of vision.
At Parker Waichman LLP, we are investigating claims nationwide on behalf of patients harmed by these recalled lenses. Our firm has built a reputation for standing up against major corporations when defective medical products put patients at risk. If you or a loved one has been diagnosed with TASS after receiving a Bausch & Lomb enVista intraocular lens, you may be entitled to financial compensation.
About the Bausch & Lomb enVista Intraocular Lenses
The Bausch & Lomb enVista intraocular lens (IOL) was marketed as an advanced solution for patients undergoing cataract removal or lens replacement surgery. These artificial lenses were designed to replace the natural lens of the eye that becomes clouded due to cataracts, with the goal of restoring clear vision and improving overall quality of life.
The enVista line included several models:
- enVista Monofocal IOL
- enVista Aspire IOL
- enVista Envy IOL
- enVista Monofocal Toric IOL
- enVista Aspire Toric IOL
- enVista Envy Toric IOL
Because cataract surgery is one of the most common procedures performed in the United States, these lenses were implanted in thousands of patients between August 2024 and April 2025. Surgeons often chose the enVista lenses for their durability, clarity, and ability to reduce unwanted visual effects such as glare and halos.
However, despite their widespread use, reports began to emerge that patients implanted with certain enVista lenses were experiencing unexpected and dangerous complications shortly after surgery. The company later revealed that the issue was linked to a raw material used in specific lots of lenses supplied by a third-party vendor. This defect directly correlated with a spike in cases of Toxic Anterior Segment Syndrome (TASS), prompting Bausch & Lomb to issue its voluntary recall.
For patients, the promise of improved sight has too often been replaced with painful symptoms, vision complications, and the need for further medical treatment. This has transformed what should have been a routine surgery into a serious health and legal matter.
FDA Recall Announcement

On April 3, 2025, Bausch & Lomb announced a voluntary recall of multiple models in its enVista intraocular lens platform in the United States. The recall was prompted by a rise in reported cases of Toxic Anterior Segment Syndrome (TASS), a rare but potentially devastating complication that can appear shortly after eye surgery.
The recall included:
- enVista Monofocal IOL (all models beginning with EE)
- enVista Aspire IOL (all models beginning with EA)
- enVista Envy IOL (all models beginning with EN)
- enVista Monofocal Toric IOL (all models beginning with ETE)
- enVista Aspire Toric IOL (all models beginning with ETA)
- enVista Envy Toric IOL (all models beginning with ETN)
According to company reports, the complication rate among these lenses was significantly higher than normal, with clusters of TASS cases being reported in late 2024 and early 2025. Although Bausch & Lomb stated that many patients responded to treatment without requiring lens removal, the seriousness of the condition and its potential for lasting harm prompted immediate regulatory attention.
The U.S. Food and Drug Administration (FDA) published the company’s recall announcement on April 7, 2025, classifying the action as a public health matter. The agency confirmed that the recall applied to all affected lots distributed in the U.S. during the implantation period of August 2024 through April 2025.
Subsequent investigations determined that a raw material used in manufacturing, supplied by a third-party vendor, contributed to the increased risk. In response, Bausch & Lomb implemented new inspection and quality control standards while simultaneously pulling the affected products from the market.
While the company has since resumed production of unaffected lenses, the damage has already been done for patients who were implanted with the recalled models. For those who developed TASS, the recall announcement serves as critical evidence linking their injuries to the defective product.
What is Toxic Anterior Segment Syndrome (TASS)?
Toxic Anterior Segment Syndrome (TASS) is a serious inflammatory reaction that can develop inside the front portion of the eye (the anterior segment) after cataract or lens replacement surgery. Unlike an infection caused by bacteria, TASS is considered a sterile inflammatory condition, meaning it results from a non-infectious contaminant or toxin that enters the eye during or after surgery. Despite this difference, the damage it causes can be just as severe as an infection if not treated promptly.
When Does TASS Occur?
TASS typically appears within 12 to 48 hours following surgery. Patients who develop the condition often notice symptoms far more quickly than those associated with infectious endophthalmitis, another post-surgical complication that usually takes several days to present.
Symptoms of TASS
Patients affected by TASS may experience:
- Significant eye pain
- Redness and swelling
- Blurred or cloudy vision
- Light sensitivity
- Severe inflammation inside the eye
- Corneal damage or corneal edema (swelling of the cornea)
In many cases, these symptoms are alarming enough to prompt an immediate return to the surgeon’s office or an emergency medical evaluation.
Long-Term Risks
While some cases of TASS can be managed with intensive steroid treatment and close monitoring, the condition can cause lasting injury to the cornea, iris, and other delicate structures of the eye. Severe cases may require:
- Corneal transplants to restore vision
- Additional surgeries to address complications
- Permanent vision loss, including blindness in the affected eye
For patients who received a Bausch & Lomb enVista lens during the affected period, the recall makes it clear that the product itself is tied to this dangerous outcome. Instead of gaining restored sight, many patients now face ongoing pain, costly medical procedures, and the possibility of irreversible damage.
The Dangers of TASS
For patients expecting clearer vision after cataract or lens replacement surgery, a diagnosis of Toxic Anterior Segment Syndrome (TASS) can be devastating. While some cases resolve with prompt treatment, others leave permanent, life-altering damage. The dangers of TASS extend far beyond temporary discomfort and can affect every aspect of a patient’s daily life.
Immediate Complications
TASS strikes quickly—typically within 12 to 48 hours of surgery. In its acute phase, the condition causes:
- Intense eye pain and pressure
- Severe inflammation
- Cloudy or blurry vision
- Extreme sensitivity to light
These symptoms often require urgent medical care and can mean repeated doctor visits, emergency procedures, and intensive steroid therapy.
Lasting Damage
Even when treated, TASS has the potential to cause irreversible harm to the eye, including:
- Corneal scarring or swelling (edema): May distort vision and reduce clarity.
- Damage to the iris or trabecular meshwork: This can increase the risk of chronic glaucoma.
- Permanent vision loss: In the most severe cases, patients may lose partial or complete sight in the affected eye.
- Need for corneal transplant or additional surgeries: To restore vision or manage long-term complications.
Impact on Daily Life
Beyond the physical damage, TASS can profoundly affect a patient’s independence, emotional health, and financial stability. Patients may struggle with basic tasks such as reading, driving, or working. Many also face the stress of ongoing medical bills, lost income from time away from work, and the fear of permanent disability.
Why This Matters for enVista Patients
The recall of the Bausch & Lomb enVista lenses highlights that these dangers were not random surgical risks but were tied to a defective product. Patients trusted that their lens implant would help restore their vision. Instead, many are left dealing with long-term medical needs and the uncertainty of whether their eyesight will ever fully recover.
At Parker Waichman LLP, we believe patients should not carry the burden of these consequences alone. Manufacturers that put defective devices on the market must be held accountable for the harm caused.
Legal Rights of Injured Patients
When a medical device like the Bausch & Lomb enVista intraocular lens causes serious complications, patients are not left without options. Under product liability law, manufacturers and distributors can be held responsible when defective devices harm those who trusted them. The recall announced in April 2025 strengthens the link between these lenses and Toxic Anterior Segment Syndrome (TASS), giving affected patients a clear legal pathway to pursue justice.
Grounds for a Claim
Patients may bring claims under several legal theories, including:
- Design or manufacturing defect: If the product was inherently unsafe due to a flawed design or the use of faulty raw materials.
- Failure to warn: If patients and surgeons were not adequately informed about potential risks.
- Negligence: If Bausch & Lomb or its suppliers failed to exercise proper care in making or distributing the lenses.
Potential Compensation
Victims of TASS caused by a defective enVista lens may be entitled to compensation for:
- Medical expenses – including surgeries, hospital stays, prescription medications, and long-term care
- Lost wages and reduced earning capacity – for time missed from work or an inability to return to employment
- Pain and suffering – for the physical discomfort, vision loss, and emotional toll caused by TASS
- Future medical needs – such as corneal transplants, ongoing monitoring, or assistive devices
- Punitive damages – in some cases, courts may award damages to punish corporations that recklessly endanger patients
Why Legal Action is Critical
The costs of treating TASS can be overwhelming, and the life changes caused by vision loss are often permanent. Pursuing a claim ensures that patients are not forced to bear these burdens alone. It also holds Bausch & Lomb accountable, sending a message that safety must come before profit.
At Parker Waichman LLP, we fight to make sure victims of defective medical devices receive full and fair compensation. Our attorneys understand the complexities of medical device litigation and have the resources to go toe-to-toe with large corporations.
Do You Have a Claim?
The Bausch & Lomb enVista intraocular lens recall is not limited to a single product—it covers multiple lens models that were implanted in thousands of patients across the United States. Understanding whether you may qualify for a legal claim starts with identifying if you meet the known case criteria.
Timeframe of Implantation
The recall applies to patients who had an enVista intraocular lens implanted between August 2024 and April 2025. This period corresponds with when the defective lots were distributed and used by surgeons across the country.
Recalled Lens Models
The recall affects the following lenses within the enVista platform:
- enVista Monofocal IOL
- enVista Aspire IOL
- enVista Envy IOL
- enVista Monofocal Toric IOL
- enVista Aspire Toric IOL
- enVista Envy Toric IOL
For simplicity, these products can collectively be referred to as Bausch & Lomb enVista intraocular lenses.
Medical Diagnosis
To meet the case criteria, patients must have been diagnosed with Toxic Anterior Segment Syndrome (TASS) following their implant surgery. TASS generally presents within the first two days after surgery and often requires urgent medical intervention.
Why These Criteria Matter
By combining the implantation timeframe, the specific lens models recalled, and a subsequent diagnosis of TASS, patients can establish a direct connection between their injuries and the defective product. This link is crucial in pursuing a successful product liability claim.
If you or a loved one fits these criteria, you may be entitled to compensation for medical expenses, lost wages, and the pain and suffering caused by the defective enVista lens. Parker Waichman LLP is actively reviewing these claims and is ready to help patients and families take legal action.
Why These Cases Are Complex
Product liability cases involving defective medical devices, such as the Bausch & Lomb enVista intraocular lenses, are far more complicated than the average personal injury claim. Patients harmed by Toxic Anterior Segment Syndrome (TASS) face a unique set of challenges when pursuing justice, which is why skilled legal representation is so important.
Proving the Connection
Even though Bausch & Lomb issued a recall, manufacturers and their insurers often argue that complications were caused by other factors—such as surgical technique, preexisting health conditions, or unrelated infections. To succeed in these cases, it is critical to gather and present evidence showing that the recalled enVista lens directly caused the injury. This often requires:
- Detailed medical records from the cataract or lens replacement surgery
- Proof of the implant model and lot number
- Expert testimony from ophthalmologists and toxicology specialists
- Scientific data linking the defective raw material to TASS outcomes
Corporate Defense Strategies
Bausch & Lomb is a global corporation with extensive resources. When patients file claims, the company will often rely on highly experienced defense teams and expert witnesses to minimize liability. Victims without legal representation risk being overpowered or pressured into accepting inadequate settlements.
Complex Medical Evidence
TASS is not widely understood outside of specialized medical circles. Building a strong case requires lawyers who know how to work with medical experts, interpret complex eye health records, and explain the science of inflammation and toxicity to judges and juries in a way that is clear and compelling.
Statutes of Limitation
Every state has strict deadlines that limit how long a patient has to bring a claim. Waiting too long could mean losing the right to compensation entirely. Acting quickly ensures that evidence is preserved and deadlines are met.
Why Parker Waichman LLP is Equipped to Handle These Challenges
Our firm has decades of experience in handling medical device recalls and mass tort litigation. We have the resources, medical networks, and trial experience needed to take on corporations like Bausch & Lomb. From gathering evidence to negotiating with insurers or going to trial, we know how to build a case that gives patients the strongest chance at recovery.
EnVista Lawsuit Frequently Asked Questions (FAQs)
Which enVista lenses were recalled, and when?
Bausch + Lomb announced a voluntary U.S. recall on April 3, 2025 covering enVista Aspire, Envy, and certain Monofocal/Toric IOLs after an uptick in Toxic Anterior Segment Syndrome (TASS) reports. The FDA posted the company announcement on April 7, 2025. Later, on April 28, 2025, the company revised the scope to specific lots and issued updated instructions to eye-care professionals.
Am I eligible for a claim?
Our current case criteria focus on patients who:
- Received a Bausch & Lomb enVista IOL (any model within the enVista platform),
- Had the lens implanted between August 2024 and April 2025, and
- Were diagnosed with TASS after surgery. If you’re unsure about your lens model or dates, we can review your surgical records and confirm. (See the model prefixes below for quick checks.)
What are the signs of TASS, and how soon does it appear?
TASS is an acute, sterile inflammatory reaction inside the front of the eye that usually presents within 12–48 hours after surgery. Common findings include decreased or blurry vision, eye pain, corneal swelling, high eye pressure, and a strong response to steroid drops.
How is TASS different from an infection like endophthalmitis?
TASS is non-infectious and typically appears faster (often 12–48 hours) than infectious endophthalmitis. It usually responds to aggressive topical steroids and lacks culture-positive growth, while an infection requires antimicrobial therapy and can present later. Your treating surgeon differentiates these conditions based on exam findings, timing, response to treatment, and testing.
Did patients need their lenses removed?
According to Bausch + Lomb’s recall announcement, the TASS cases they received responded quickly to treatment, and none required lens removal. Individual care decisions are medical calls made by your surgeon, but the company’s statement is important context for claims.
What caused the increase in TASS reports?
Following a company investigation with outside advisors, Bausch + Lomb reported the issue stemmed from raw material used in certain lots supplied by a vendor. The company said it implemented enhanced inspection protocols and clarified standards for lens monomers; it then moved to return unaffected lots to market.
Are enVista lenses back on the market now?
Yes—after identifying the source and confirming the affected lots, Bausch + Lomb announced plans to return unaffected lots to market and ramp production back to full U.S. supply in the following weeks (late April 2025). Trade coverage echoed the rapid resupply timeline.
How can I tell which model I received?
Ask your surgeon or surgical center for your operative report, implant sticker/labels, and UDI (unique device identifier). Helpful model prefixes listed in FDA recall records include:
- EE (enVista Monofocal), EA (Aspire), EN (Envy), ETE (Monofocal Toric), ETA (Aspire Toric), ETN (Envy Toric).
- These are a quick way to confirm the model while we gather the full documentation.
What evidence should I keep or collect?
- Surgical/implant records (model, lot/serial, UDI)
- Office notes confirming TASS diagnosis and treatment timeline
- Bills and pharmacy receipts
- Any work-impact records (lost wages, accommodations)
- If an explant ever occurs, the device should be preserved and documented rather than discarded. We handle evidence preservation for clients.
- Any other records/documents you may have in your possession potentially related to your claim.
What compensation could be available?
Potential damages include medical costs (past and future), lost wages/earning capacity, pain and suffering, and future care (e.g., corneal procedures, ongoing monitoring). In limited circumstances, courts may consider punitive damages. The value of any claim depends on medical proof and the impact on your life.
How long do I have to file?
Deadlines (statutes of limitation and repose) vary by state and can be affected by the date of surgery, the date you discovered the injury, and recall disclosures. Because time can run quickly—and records are easier to secure earlier—we recommend starting a review now so you don’t lose your rights.
Does the recall mean I automatically get paid?
No. A recall is powerful evidence, but compensation isn’t automatic. You still need to show that a recalled or affected enVista lens was implanted and that it caused your TASS injury and losses. You will also need to establish a case against the manufacturer and prove that they should be held liable for your injuries. That’s what our legal team builds with medical and technical experts.
Can I bring a claim if my symptoms improved with treatment?
Yes. Even if acute inflammation resolved, you may still have compensable harms such as residual vision changes, corneal damage, high IOP/glaucoma risk, or economic losses.
Could my surgeon be at fault?
Most claims focus on the manufacturer and supply chain when a defective product or raw material is implicated. In rare circumstances, separate medical-negligence issues could exist, but those are evaluated case-by-case after we review your records and the recall scope.
What if my surgery was outside the August 2024–April 2025 window, or I’m not sure about dates?
Start by getting your operative report and implant stickers. If your surgery occurred slightly outside the window, we’ll still review—particularly if you had TASS within 12–48 hours and a lens model or lot overlaps with recall notices or later scope updates. If your implant surgery was significantly outside the window (e.g. more than 2 months before or after) it is unlikely that you will qualify.
What’s the process with Parker Waichman LLP?
- Free case review and records request (we help obtain your medical/implant documentation).
- Medical and technical evaluation to confirm device model/lot, timing, and TASS diagnosis.
- Claim filing and negotiations with the manufacturer/insurers; litigation if needed.
- We handle the heavy lifting so you can focus on your health.
How much will this cost me?
We work on a contingency-fee basis: No fees unless we recover money for you. You pay nothing out of pocket for our services.
If you meet the criteria—or even if you’re unsure—call 800-968-7529 for a free, no-obligation review.
How Parker Waichman LLP Can Help
When a medical device recall disrupts lives, patients need more than just answers. They need an advocate who can take on a corporation and demand accountability. At Parker Waichman LLP, we have built a national reputation for representing individuals harmed by defective drugs, implants, and medical devices. Our attorneys understand how to guide patients and families through these complex cases and fight for the compensation they deserve.
Investigating Your Case
Our legal team will immediately begin by:
- Reviewing your surgical and medical records to confirm whether a recalled enVista lens was implanted.
- Consulting with ophthalmologists and medical experts to confirm the diagnosis of TASS.
- Preserving evidence that may prove the connection between the recalled lens and your injuries.
Building a Strong Legal Claim
We know that Bausch & Lomb will rely on experienced defense teams. That’s why we use our own network of respected medical experts, engineers, and product safety specialists to build the strongest possible case. This includes developing testimony, scientific data, and documentation that clearly explains how the defective lens caused harm.
Handling Every Step for You
Parker Waichman LLP takes care of every detail of your claim. We handle the paperwork, communications with insurers and opposing counsel, and court filings, allowing you to focus on recovery. Our attorneys are experienced in negotiating favorable settlements and are fully prepared to go to trial if necessary.
A Track Record of Results
Parker Waichman LLP is part of a select group of law firms that has recovered more than $2 billion in verdicts and settlements for clients. Our history shows that we don’t back down from large corporations, and we know how to win cases that matter for patients and families.
If you or someone you love developed TASS after being implanted with a recalled enVista intraocular lens, our firm is ready to fight for you.
Why Choose Parker Waichman LLP
Choosing the right law firm can make the difference between a frustrating experience and a successful recovery. At Parker Waichman LLP, we are committed to standing by patients harmed by defective medical devices like the recalled Bausch & Lomb enVista intraocular lenses. Our reputation has been built on results, trust, and decades of experience fighting for those injured by corporate negligence.
No Recovery, No Fee
We represent clients on a contingency-fee basis, which means you pay nothing up front. Our fees are collected only if we secure compensation for you through a settlement or jury verdict. If we don’t win, you don’t pay.
National Recognition
Our firm has been consistently recognized by peers, judges, and independent rating organizations for excellence in personal injury litigation. We have earned:
- A near-perfect AVVO rating
- The highest peer-review rating from Martindale-Hubbell
- Recognition in Best Lawyers
- Lawdragon’s top rating of “5 Dragons”
Decades of Legal Experience
Our attorneys have successfully represented thousands of clients in personal injury and product liability claims in both state and federal courts. We know how to handle complex litigation involving defective medical products and how to hold corporations accountable for their mistakes.
Respected by Clients and Peers
The trust we have built is not only with those we represent but also with fellow attorneys, judges, and even opposing counsel. Our reputation reflects our commitment to professional excellence and tireless advocacy for our clients.
A Record of Results
With over $2 billion recovered in verdicts and settlements, Parker Waichman LLP has demonstrated time and again that we have the resources and determination to win high-stakes cases.
When your health, vision, and future are on the line, you deserve a law firm with proven results and the ability to fight against one of the largest medical device manufacturers in the world.
Protect Your Claim, Take Action Today.
If you or someone you love developed Toxic Anterior Segment Syndrome (TASS) after cataract or lens replacement surgery with a recalled Bausch & Lomb enVista intraocular lens, you may have the right to pursue significant compensation. These devices were meant to restore vision, not cause pain, inflammation, and long-term damage. Patients should not be left to carry the burden of medical expenses, lost income, and life-altering complications caused by a defective product.
At Parker Waichman LLP, we are actively reviewing cases nationwide. Our attorneys have the knowledge, resources, and proven track record to hold Bausch & Lomb accountable and fight for the justice you deserve.
- Free Case Evaluation: We will review your medical history, confirm whether you received a recalled lens, and explain your legal options at no cost.
- No Fees Unless You Win: You will never pay out of pocket. We only collect a fee if we secure a settlement or verdict for you.
- Nationwide Representation: No matter where you are in the United States, our firm is prepared to assist you.
CONTACT Parker Waichman LLP FOR A FREE CASE REVIEW
Don’t wait. Time limits apply to product liability claims. Protect your rights and take the first step toward justice. Call 800-968-7529 today or fill out our online consultation form to speak with a Parker Waichman LLP attorney.