How To Obtain Full Compensation For Equinoxe Shoulder Systems Device Degradation or Malfunction Post-Implantation
The Equinoxe Shoulder System joint replacement devices, manufactured by Exactech between 2004 and August 2021, have been flagged by the FDA due to potential health hazards stemming from defective packaging. These devices, packaged in faulty bags lacking crucial oxygen barrier layers, pose significant risks such as accelerated device wear, fracture, failure, and associated complications like pain, bone loss, and swelling. Equinoxe shoulder replacements are among a long list of critically flawed Exactech products.
If you or a loved one has suffered Equinoxe Shoulder Systems device degradation or malfunction post-implantation, it’s crucial to understand how to obtain full compensation for your losses.
Parker Waichman LLP, a top-rated national product liability law firm has recovered over $2 billion in compensation for our clients who have been injured or lost loved ones due to defective products.
Parker Waichman LLP is offering free consultations and is currently representing claimants in Equinoxe Shoulder Systems lawsuits throughout the United States. These lawsuits seek to recover compensation for those harmed by device degradation or malfunction that has caused severe complications, including injuries, impairment, and corrective surgery. Contact us today at 800-968-7529 to discuss your situation and learn more about how we can help you obtain the economic compensation your case deserves.
What Are Exactech Equinoxe Shoulder Systems?
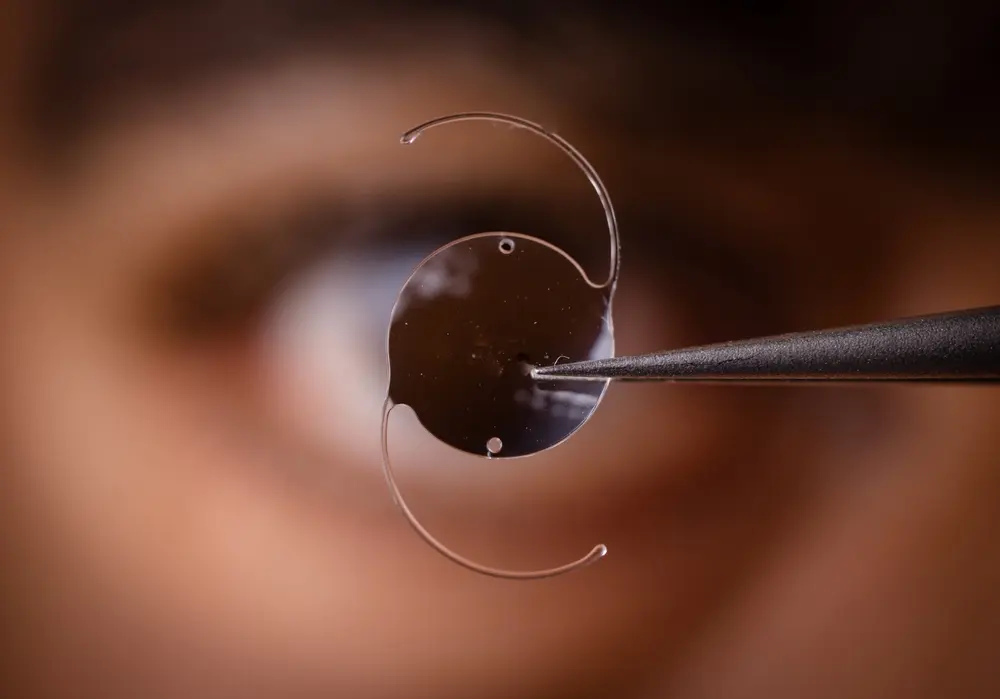
The Equinoxe Shoulder System is a series of medical devices designed for shoulder joint replacement procedures, primarily aimed at alleviating pain and improving function in patients suffering from conditions such as osteoarthritis, osteonecrosis, rheumatoid arthritis, and structural deformities. Developed by Exactech, these systems have been utilized in adult patients experiencing significant shoulder joint deterioration or failure, either as a result of degenerative diseases or previous unsuccessful joint replacement surgeries.
The Equinoxe Shoulder Systems consist of various components, including humeral heads, glenoid components, stems, and other associated instruments, allowing orthopedic surgeons to tailor the implantation process according to each patient’s specific needs and anatomical characteristics.
These systems are engineered to mimic the natural movement and function of the shoulder joint, restoring mobility and enhancing overall quality of life for individuals suffering from debilitating shoulder conditions.
One of the key features of the Equinoxe Shoulder Systems is their versatility and adaptability to different patient anatomies and pathologies. With a range of sizes, configurations, and surgical techniques available, orthopedic surgeons can select the most appropriate components to optimize surgical outcomes and patient satisfaction. Additionally, the Equinoxe Shoulder Systems incorporate advanced materials and design principles to promote long-term durability and stability, essential factors in ensuring the success of shoulder replacement procedures.
The U.S. FDA Issues a Safety Communication
The U.S. Food and Drug Administration (FDA) issued a safety communication on January 16, 2024, regarding potential health risks associated with the Equinoxe Shoulder System joint replacement devices manufactured by Exactech between 2004 and August 2021. These devices were packaged in defective bags, which could lead to oxidation of the plastic components over time, posing risks such as device wear, fracture, failure, pain, bone loss, swelling, and the need for revision surgery.
Patients with well-functioning Equinoxe Shoulder Systems and no symptoms are not recommended to undergo surgery. However, those experiencing pain, swelling, arm weakness, or unusual noises around the implanted device should contact their healthcare provider.
Health care providers are advised not to implant any Equinoxe Shoulder Systems packaged in defective bags and to monitor patients for potential device-related issues, considering X-rays if device failure is suspected.
Equinoxe Shoulder System implants are used in adults to replace painful shoulder joints due to various conditions, including osteoarthritis and rheumatoid arthritis. Defective packaging, missing an oxygen barrier layer, can allow oxygen to contact the plastic components before implantation, leading to oxidation and subsequent complications.
Patients experiencing problems with their devices are encouraged to report them through the FDA’s MedWatch Voluntary Reporting Form. The Unique Device Identifier (UDI) system helps identify individual medical devices, facilitating more accurate reporting and analysis of adverse events. An affected device can be identified by checking the UDI on its label.
Despite the FDA’s alert, Exactech has not initiated a voluntary recall for the affected implants. The FDA continues to collaborate with Exactech and international regulatory agencies to evaluate the safety and effectiveness of joint replacement devices containing plastic components packaged in defective bags. Updates will be provided as significant information becomes available.
Recent Developments on Exactech Equinoxe Shoulder Systems Recall and Litigation
Exactech, a medical device manufacturer specializing in joint replacement implants, has faced significant challenges due to defective packaging of its Equinoxe Shoulder Systems. These issues have led to recalls, extensive litigation, and ultimately, the company’s bankruptcy filing.
FDA Safety Communication and Recall
In January 2024, the U.S. Food and Drug Administration (FDA) issued a safety communication regarding Exactech’s Equinoxe Shoulder Systems manufactured between 2004 and August 2021. The FDA highlighted that these devices were packaged in defective bags lacking an essential oxygen barrier layer, leading to potential oxidation of the plastic components. This oxidation could result in accelerated device wear, component fractures, and overall device failure, necessitating revision surgeries for affected patients.
Initially, Exactech declined to initiate a voluntary recall for the affected shoulder implants. However, following the FDA’s communication and growing concerns, the company proceeded with a recall to remove these devices from the market.
Litigation and Bankruptcy Filing
The defective packaging and subsequent device failures led to approximately 2,600 lawsuits against Exactech. Patients alleged harm from the faulty implants, citing issues such as pain, swelling, bone loss, and the need for corrective surgeries. The financial strain from these legal battles, coupled with existing debts, culminated in Exactech filing for bankruptcy in October 2024.
Impact on Patients and Healthcare Providers
The recall has significantly impacted patients who received the defective implants. Many have experienced complications requiring additional medical interventions. Healthcare providers have been advised to monitor patients with these implants closely and consider revision surgeries if symptoms of device failure are present.
Frequently Asked Questions (FAQs) on Exactech Equinoxe Shoulder Systems Recall
What is the Exactech Equinoxe Shoulder System?
The Exactech Equinoxe Shoulder System is a medical device designed for shoulder joint replacement surgeries. It aims to alleviate pain and restore function in patients suffering from conditions like osteoarthritis, osteonecrosis, rheumatoid arthritis, and structural deformities. The system comprises components such as humeral heads, glenoid components, and stems, allowing orthopedic surgeons to customize the implantation based on individual patient needs.
Why was there a recall of the Equinoxe Shoulder System?
The recall was initiated due to defective packaging of the implants. The packaging lacked a crucial oxygen barrier layer, leading to oxidation of the plastic components before implantation. This oxidation could cause accelerated device wear, fractures, and overall failure, resulting in complications like pain, bone loss, and the necessity for revision surgeries.
How do I know if my shoulder implant is affected by the recall?
If you underwent shoulder replacement surgery between 2004 and August 2021 using the Exactech Equinoxe Shoulder System, your implant might be affected. It’s essential to consult your orthopedic surgeon or healthcare provider to determine if your implant is among the recalled devices. They can provide specific information based on your medical records and guide you on the necessary steps.
What symptoms should I watch for if I have an affected implant?
Patients with the recalled implants should be vigilant for symptoms indicating potential device failure, including:
- New or worsening pain in the shoulder
- Swelling around the implant site
- Decreased range of motion or inability to use the arm
- Grinding or unusual noises from the shoulder
- Weakness in the shoulder or arm
If you experience any of these symptoms, contact your healthcare provider promptly for evaluation.
What should I do if my implant is functioning well without any symptoms?
The FDA does not recommend proactive removal of well-functioning implants in asymptomatic patients. However, regular follow-ups with your healthcare provider are crucial to monitor the implant’s condition and ensure any potential issues are detected early.
What are the legal options available for affected patients?
Patients adversely affected by the defective implants may be entitled to compensation for medical expenses, pain and suffering, lost wages, and other related damages. It’s advisable to consult with a legal professional experienced in medical device litigation to explore your options and understand the process of filing a claim.
How does Exactech’s bankruptcy filing affect ongoing lawsuits and potential compensation?
Exactech’s bankruptcy filing introduces complexities to the litigation process. While the company seeks to restructure its debts and address the pending lawsuits, the timeline and availability of compensation for affected patients may be impacted. Staying informed through legal counsel is essential to navigate this evolving situation effectively.
Are other Exactech implants affected by similar issues?
Yes, Exactech has faced recalls for other joint replacement devices, including certain knee, ankle, and hip implants, due to similar packaging defects leading to oxidation and premature device failure. Patients with these implants should consult their healthcare providers to assess any potential risks.
What steps should healthcare providers take concerning the recalled implants?
Healthcare providers are advised to:
- Cease implantation of any Equinoxe Shoulder Systems packaged in defective bags.
- Monitor patients with the affected implants for signs of device wear, failure, or bone loss.
- Conduct imaging studies, such as X-rays, if device failure is suspected.
What compensation can I receive if I file a lawsuit for an Exactech Equinoxe Shoulder System failure?
If you or a loved one experienced complications due to an Exactech Equinoxe Shoulder System failure, you may be entitled to financial compensation. The amount and type of compensation depend on the severity of your injuries, medical expenses, and other factors. Potential damages in these lawsuits include:
- Medical Expenses: Reimbursement for past and future medical treatments, including revision surgeries, physical therapy, medications, and hospital stays related to the defective implant.
- Pain and Suffering: Compensation for the physical pain and emotional distress caused by device failure, loss of mobility, and chronic discomfort from the implant.
- Lost Wages: If your injuries prevented you from working or caused a temporary or permanent disability, you may recover lost wages and future earning capacity.
- Disability and Impairment: If the device failure led to permanent disability, reduced mobility, or loss of function, you may be entitled to higher compensation.
- Punitive Damages: In some cases, if it is proven that Exactech knew about the defect and failed to act, courts may award punitive damages to punish the company for negligence.
Exact settlement amounts vary based on individual cases, but many patients have filed lawsuits seeking six- and seven-figure settlements for medical costs, pain, suffering, and long-term health effects. If you have been affected, consulting an experienced product liability attorney can help you determine your legal options and maximize your potential compensation.
Contact Parker Waichman LLP for a Free Case Review
Families affected by a defective medical implant should contact a defective medical device attorney immediately. Contact us by calling 800-968-7529 for a free case review. Regardless of your location or where your injury occurred, our nationwide law firm is ready to assist you.